Maybe you’ve been just like myself, too tired to be surprised or very concerned with the new wave of magneto-vaxxers. But we have to make the effort to take this as it most likely is: super-serious.
UPDATE JULY 2021: GRAPHENE OXYDE CONFIRMED AS MAIN INGREDIENT (99%) IN THE PFIZER VACCINE. AMONG OTHER DESASTRUOUS EFFECTS: BLOOD-CLOTTING and covid symptoms. HOW DID WE GET TO THIS REVELATION? READ THE FIRST INVESTIGATION INTO MAGNETOGENETICS EVER!

La Quinta Columna has recently made an urgent announcement that they hope will reach as many people as possible, especially those involved in health and legal services, as biostatistician Ricardo Delgado, Dr. José Luis Sevillano and the team of researchers and professors with whom they have been conducting their research have confirmed the presence of graphene oxide nanoparticles in vaccination vials.
In program nº63, the team showed some photos of the analyses carried out, specifically results obtained by optical and transmission electron microscopy observation, reserving the results of other techniques used for future programs. They also announced that the report based on all the techniques performed, which allowed determining the presence of graphene oxide, will be made official by the researchers who performed the analyses very soon….
There’s indications, if not full fledge proof, that masks being used and currently marketed contain graphene oxide. Not only the ones that were withdrawn at the time, as indicated by the media, the swabs used in both PCR and antigen tests also contain graphene oxide nanoparticles.
Graphene oxide can generates blood coagulation. Graphene oxide causes alteration of the immune system. By decompensating the oxidative balance in relation to the gulation reserves. If the dose of graphene oxide is increased by any route of administration, it causes the collapse of the immune system and subsequent cytokine storm.
Graphene oxide accumulated in the lungs generates bilateral pneumonias by uniform dissemination in the pulmonary alveolar tract. Graphene oxide causes a metallic taste. Perhaps this is starting to make sense to you now. Inhaled graphene oxide causes inflammation of the mucous membranes and thus loss of taste and partial or total loss of smell.
Graphene oxide acquires powerful magnetic properties inside the organism. This is the explanation for the magnetic phenomenon that billions of people around the world have already experienced after various routes of administration of graphene oxide. Among them the vaccine….
It is therefore absolutely essential and vital that you make this information available to your medical community.
Meanwhile, I found this total match:

What they haven’t found yet is these new kids on the block, Made in China and with racial implications, all the due triggers are packed in:



A bit later:
Inbrain is just Merck now.
As I said many times, Big Pharma and Big Tech are dead, long live The Great Military BioTech Complex!

When evidence is overwhelming, official confirmation is not as relevant, though. We’re kinda there.
URGENT! IT’S IN MASKS TOO: SUPER-TOXIC GRAPHENE OXIDE CONFIRMED BY MANUFACTURERS

And then:
GET READY FOR SMART WATER: GRAPHENE OXIDE FOR WATER SUPPLY QUALITY CONTROL
So you have precisely 0 (zero) reasons for surprise if they find some type of graphene fibers in masks or swabs.
Older studies on graphene oxide below, now let’s see how we got here.
It all started with the courageous or desperate Pharma-dupes who got jabbed (yeah, vaxxers started it), and then had the guts to expose themselves on Internet with their weird magnetic symptoms. They got followed by courageous and persistent citizen-journalists like Tim Truth, who managed to capture attention from journalists like myself and a few others with bigger or smaller platforms, who, together, managed to push this as mainstream as Jimmy Kimmel.
BREAKING:
The scientific method to debunk an experiment is to repeat it. If they really were serious about debunking it, they would’ve given everyone free magnets to test it. Instead…
UPDATE: SOMETHING BIG IS HAPPENING! Youtube censorship entered overdrive, they’ve deleted us a second video on this in 24h, this time it was THE Ben Swann investigation on DARPA’S MAGNETIC MIND CONTROL PROGRAM. both my channels are hanging on a thread now.
PLEASE SPREAD THIS INFO LIKE FIRE!
i’M NOT SAYING “SPREAD MY LINK,” BUT SPREAD THE INFO THAT SOUNDS RIGHT TO YOU AND DO IT NOW!
OUR SECOND VIDEO DELETED BY YOUTUBE IN ONE DAY!
Proof that this is serious: as I was wrapping this report up, YouTube has just deleted my COMEDY take on this, proving that we struck a chord.
update #2: more examples of “magnetovaxxers” found and compiled
More and more people are taking the magnet challenge
Thanks Tim Truth for sparking my investigation and following up!
HERE you can watch his latest compilation of vaxxers turning into fridge doors
If next minute all vaxxtards turn into transformer drones, I’m not going to be very surprised, rather amused. But i should be concerned.
I am concerned with sticky vaxxers because most likely there’s some magnetogenetics involved. It’s almost impossible that this is not the explanation for the new Internet sensation.
Earliest academic mention of magnetogenetics I found comes from China, but in the meantime I’ve learned this goes back to 2010 and beyond, more updates soon:
UPDATE #3
in just hours, youtube admits my appeal and reinstates the Ben Swann Video!
Can hardly keep up with myself lol
What did the appeal say that was unprecedently persuasive?
I don’t have the exact words, but the main ideas were:
1. Everything you’ve just claim is a lie, it’s offensive and defaming, but that’s ok because your words have no value.
2. Thanks for pointing out you’re especially sensitive about this topic, we’ll put it on turbo-boost!
UPDATE #4 mAY 20 2021: WE’VE ALREADY WON THE INFORMATION WAR AGAINST BIG TECH, THE KNOWLEDGE IS MAINSTREAM NOW, ICKE AND THE LAST AMERICAN VAGABOND ALL OVER IT
NOW ENTER THE SCIENCE
Nikhil Hajirnis tells us how cells developed strategies to detect light and magnetism with evolution of a class of proteins called the crytochromes. And now we use this understanding to alter magnetic fields around cells to image cells as well as to attempt changing the way they work.
Source: CSIR – Centre For Cellular And Molecular Biology
Magnetogenetics: remote non-invasive magnetic activation of neuronal activity with a magnetoreceptor
Source: https://doi.org/10.1007/s11434-015-0902-0
Abstract
Current neuromodulation techniques such as optogenetics and deep-brain stimulation are transforming basic and translational neuroscience. These two neuromodulation approaches are, however, invasive since surgical implantation of an optical fiber or wire electrode is required. Here, we have invented a non-invasive magnetogenetics that combines the genetic targeting of a magnetoreceptor with remote magnetic stimulation. The non-invasive activation of neurons was achieved by neuronal expression of an exogenous magnetoreceptor, an iron-sulfur cluster assembly protein 1 (Isca1). In HEK-293 cells and cultured hippocampal neurons expressing this magnetoreceptor, application of an external magnetic field resulted in membrane depolarization and calcium influx in a reproducible and reversible manner, as indicated by the ultrasensitive fluorescent calcium indicator GCaMP6s. Moreover, the magnetogenetic control of neuronal activity might be dependent on the direction of the magnetic field and exhibits on-response and off-response patterns for the external magnetic field applied. The activation of this magnetoreceptor can depolarize neurons and elicit trains of action potentials, which can be triggered repetitively with a remote magnetic field in whole-cell patch-clamp recording. In transgenic Caenorhabditis elegans expressing this magnetoreceptor in myo-3-specific muscle cells or mec-4-specific neurons, application of the external magnetic field triggered muscle contraction and withdrawal behavior of the worms, indicative of magnet-dependent activation of muscle cells and touch receptor neurons, respectively. The advantages of magnetogenetics over optogenetics are its exclusive non-invasive, deep penetration, long-term continuous dosing, unlimited accessibility, spatial uniformity and relative safety. Like optogenetics that has gone through decade-long improvements, magnetogenetics, with continuous modification and maturation, will reshape the current landscape of neuromodulation toolboxes and will have a broad range of applications to basic and translational neuroscience as well as other biological sciences. We envision a new age of magnetogenetics is coming. – Copyright © 2015 Science China Press. Published by Elsevier B.V.
CHINA FOLLOWED ALMOST SHOULDER TO SHOULDER BY DARPA
Missed DARPA?
06 Oct 2015 | 15:29 GMT
DARPA Wants to Jolt the Nervous System with Electricity, Lasers, Sound Waves, and Magnets
The defense agency announces funding for 7 projects under its new ElectRx program
By Spectrum
Viewing the body as a chemical system and treating maladies with pharmaceuticals is so 20th century. In 21st century medicine, doctors may consider the body as an electrical system instead, and prescribe therapies that alter the electrical pulses that run through the nerves.
That’s the premise of DARPA’s newest biomedical program, anyway. The ElectRx program aims to treat disease by modulating the activity of the peripheral nerves that carry commands to all the organs and muscles of the human body, and also convey sensory information back to the brain.
Yesterday, DARPA announced the first seven grants under the ElectRx program. The scientists chosen are doing fairly fundamental research, because we’re still in the early days of electric medicine; they’ll investigate mechanisms by which to stimulate the nerves, and map nerve pathways that respond to that stimulation. They’re working on treatments for disorders such as chronic pain, post-traumatic stress, and inflammatory bowel disease.
The proposed stimulation methods are fascinating in their diversity. Researchers will not only stimulate nerves with jolts of electricity, they’ll also use pulses of light, sound waves, and magnetic fields.
Three research teams using electrical stimulation will target the vagus nerve, which affects many different parts of the body. IEEE Spectrum explored the medical potential of vagus nerve hacking in a recent feature article, writing:
Look at an anatomy chart and the importance of the vagus nerve jumps out at you. Vagus means “wandering” in Latin, and true to its name, the nerve meanders around the chest and abdomen, connecting most of the key organs—heart and lungs included—to the brain stem. It’s like a back door built into the human physiology, allowing you to hack the body’s systems.
The light-based stimulation research comes from the startup Circuit Therapeutics. The company was cofounded by Stanford’s Karl Deisseroth, one of the inventors of optogenetics, the new technique that inserts light-sensitive proteins into neurons and then uses pulses of light to turn those neurons “on” and “off.” Under the DARPA grant, the researchers will try to use pulses of light to alter neural circuits involved in neuropathic pain.
To tweak the nervous system with sound waves, Columbia University’s Elisa Konofagou will use a somewhat mysterious ultrasound technique. In an e-mail, Konofagou explains that it’s already known that ultrasound can be used to stimulate neurons, but with the DARPA grant, she hopes to figure out how it works. Her hypothesis: As ultrasound propogates through biological tissue, it exerts mechanical pressure on that tissue, which stimulates specific mechanosensitive channels in neurons and causes them to “turn on.”
The final project will rely on magnetic fields to activate neurons, using a technique that could be called “magnetogenetics.” An MIT team led by Polina Anikeeva will insert heat-sensitive proteins into neurons, and will then deploy magnetic nanoparticles that bind to the surface of those neurons. When exposed to a magnetic field, these nanoparticles heat up and activate the neurons to which they’re attached.
Figuring out how to alter the activity of the nervous systems with these various tricks will be a pretty impressive accomplishment. But in the DARPA world, achieving that understanding is just step one. Next, the agency wants its grantees to develop “closed-loop” systems capable of detecting biomarkers that signal the onset of disease, and then respond automatically with neural stimulation. Spectrum covered the first such closed-loop neural stimulators in a recent feature article, stating:
The goal of all these closed-loop systems is to let doctors take their expert knowledge—their ability to evaluate a patient’s condition and adjust therapy accordingly—and embed it in an implanted device.
– Spectrum
I bet all that goes great served with some trans-cranial magnetic brainwashing.
Military magnetic field breakthrough could lead to mind reading computers and Harry Potter ‘wands’ to check for head injuries
- DARPA’s new project aims to focus on detecting superweak magnetic fields
- The research could let medics rapidly diagnose concussions on the battlefield
- It could also lead to brain-machine interfaces for controlling prosthetic limbs and external machines through the magnetic signals associated with thought
By CECILE BORKHATARIA FOR DAILYMAIL.COM
PUBLISHED: 22:35 BST, 20 March 2017 | UPDATED: 22:35 BST, 20 March 2017
Our own body generates electric currents that create ripples in the surrounding magnetic field.
These magnetic field variations allow medical professionals to use certain diagnostic tools for brain and heart conditions.
But now new research led by DARPA (Defense Advanced Research Projects Agency) aims to go beyond these diagnostic tests and develop magnetic field sensing for broader applications such as brain-machine interfaces (BMIs) for uses such as controlling prosthetic limbs and external machines through the magnetic signals associated with thought.

SOURCE
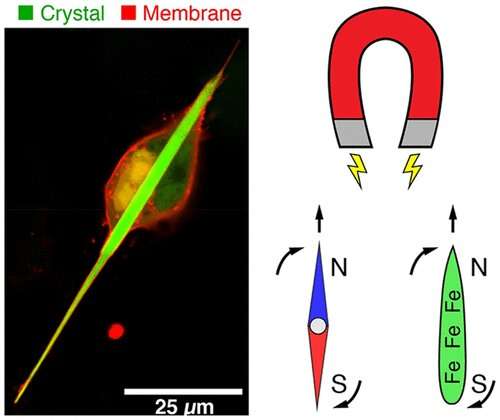
Engineered protein crystals make cells magnetic

If scientists could give living cells magnetic properties, they could perhaps manipulate cellular activities with external magnetic fields. But previous attempts to magnetize cells by producing iron-containing proteins inside them have resulted in only weak magnetic forces. Now, researchers reporting in ACS’ Nano Letters have engineered genetically encoded protein crystals that can generate magnetic forces many times stronger than those already reported.
The new area of magnetogenetics seeks to use genetically encoded proteins that are sensitive to magnetic fields to study and manipulate cells. Many previous approaches have featured a natural iron-storage protein called ferritin, which can self-assemble into a “cage” that holds as many as 4,500 iron atoms. But even with this large iron-storage capacity, ferritin cages in cells generate magnetic forces that are millions of times too small for practical applications. To drastically increase the amount of iron that a protein assembly can store, Bianxiao Cui and colleagues wanted to combine the iron-binding ability of ferritin with the self-assembly properties of another protein, called Inkabox-PAK4cat, that can form huge, spindle-shaped crystals inside cells. The researchers wondered if they could line the hollow interiors of the crystals with ferritin proteins to store larger amounts of iron that would generate substantial magnetic forces.
To make the new crystals, the researchers fused genes encoding ferritin and Inkabox-PAK4cat and expressed the new protein in human cells in a petri dish. The resulting crystals, which grew to about 45 microns in length (or about half the diameter of a human hair) after 3 days, did not affect cell survival. The researchers then broke open the cells, isolated the crystals and added iron, which enabled them to pull the crystals around with external magnets. Each crystal contained about five billion iron atoms and generated magnetic forces that were nine orders of magnitude stronger than single ferritin cages. By introducing crystals that were pre-loaded with iron to living cells, the researchers could move the cells around with a magnet. However, they were unable to magnetize the cells by adding iron to crystals already growing in cells, possibly because the iron levels in cells were too low. This is an area that requires further investigation, the researchers say.
Genetically engineered ‘Magneto’ protein remotely controls brain and behaviour
“Badass” new method uses a magnetised protein to activate brain cells rapidly, reversibly, and non-invasively
THE GUARDIAN, Thu 24 Mar 2016 14.30 GMT
Researchers in the United States have developed a new method for controlling the brain circuits associated with complex animal behaviours, using genetic engineering to create a magnetised protein that activates specific groups of nerve cells from a distance.
Understanding how the brain generates behaviour is one of the ultimate goals of neuroscience – and one of its most difficult questions. In recent years, researchers have developed a number of methods that enable them to remotely control specified groups of neurons and to probe the workings of neuronal circuits.
The most powerful of these is a method called optogenetics, which enables researchers to switch populations of related neurons on or off on a millisecond-by-millisecond timescale with pulses of laser light. Another recently developed method, called chemogenetics, uses engineered proteins that are activated by designer drugs and can be targeted to specific cell types.
Although powerful, both of these methods have drawbacks. Optogenetics is invasive, requiring insertion of optical fibres that deliver the light pulses into the brain and, furthermore, the extent to which the light penetrates the dense brain tissue is severely limited. Chemogenetic approaches overcome both of these limitations, but typically induce biochemical reactions that take several seconds to activate nerve cells.
The new technique, developed in Ali Güler’s lab at the University of Virginia in Charlottesville, and described in an advance online publication in the journal Nature Neuroscience, is not only non-invasive, but can also activate neurons rapidly and reversibly.
Several earlier studies have shown that nerve cell proteins which are activated by heat and mechanical pressure can be genetically engineered so that they become sensitive to radio waves and magnetic fields, by attaching them to an iron-storing protein called ferritin, or to inorganic paramagnetic particles. These methods represent an important advance – they have, for example, already been used to regulate blood glucose levels in mice – but involve multiple components which have to be introduced separately.
The new technique builds on this earlier work, and is based on a protein called TRPV4, which is sensitive to both temperature and stretching forces. These stimuli open its central pore, allowing electrical current to flow through the cell membrane; this evokes nervous impulses that travel into the spinal cord and then up to the brain.
Güler and his colleagues reasoned that magnetic torque (or rotating) forces might activate TRPV4 by tugging open its central pore, and so they used genetic engineering to fuse the protein to the paramagnetic region of ferritin, together with short DNA sequences that signal cells to transport proteins to the nerve cell membrane and insert them into it.
In vivo manipulation of zebrafish behavior using Magneto. Zebrafish larvae exhibit coiling behaviour in response to localized magnetic fields. From Wheeler et al (2016).
When they introduced this genetic construct into human embryonic kidney cells growing in Petri dishes, the cells synthesized the ‘Magneto’ protein and inserted it into their membrane. Application of a magnetic field activated the engineered TRPV1 protein, as evidenced by transient increases in calcium ion concentration within the cells, which were detected with a fluorescence microscope.
Next, the researchers inserted the Magneto DNA sequence into the genome of a virus, together with the gene encoding green fluorescent protein, and regulatory DNA sequences that cause the construct to be expressed only in specified types of neurons. They then injected the virus into the brains of mice, targeting the entorhinal cortex, and dissected the animals’ brains to identify the cells that emitted green fluorescence. Using microelectrodes, they then showed that applying a magnetic field to the brain slices activated Magneto so that the cells produce nervous impulses.
To determine whether Magneto can be used to manipulate neuronal activity in live animals, they injected Magneto into zebrafish larvae, targeting neurons in the trunk and tail that normally control an escape response. They then placed the zebrafish larvae into a specially-built magnetised aquarium, and found that exposure to a magnetic field induced coiling manouvres similar to those that occur during the escape response. (This experiment involved a total of nine zebrafish larvae, and subsequent analyses revealed that each larva contained about 5 neurons expressing Magneto.)
In one final experiment, the researchers injected Magneto into the striatum of freely behaving mice, a deep brain structure containing dopamine-producing neurons that are involved in reward and motivation, and then placed the animals into an apparatus split into magnetised a non-magnetised sections. Mice expressing Magneto spent far more time in the magnetised areas than mice that did not, because activation of the protein caused the striatal neurons expressing it to release dopamine, so that the mice found being in those areas rewarding. This shows that Magneto can remotely control the firing of neurons deep within the brain, and also control complex behaviours.
Neuroscientist Steve Ramirez of Harvard University, who uses optogenetics to manipulate memories in the brains of mice, says the study is “badass”.
“Previous attempts [using magnets to control neuronal activity] needed multiple components for the system to work – injecting magnetic particles, injecting a virus that expresses a heat-sensitive channel, [or] head-fixing the animal so that a coil could induce changes in magnetism,” he explains. “The problem with having a multi-component system is that there’s so much room for each individual piece to break down.”
“This system is a single, elegant virus that can be injected anywhere in the brain, which makes it technically easier and less likely for moving bells and whistles to break down,” he adds, “and their behavioral equipment was cleverly designed to contain magnets where appropriate so that the animals could be freely moving around.”
‘Magnetogenetics’ is therefore an important addition to neuroscientists’ tool box, which will undoubtedly be developed further, and provide researchers with new ways of studying brain development and function.
Reference
Wheeler, M. A., et al. (2016). Genetically targeted magnetic control of the nervous system. Nat. Neurosci., DOI: 10.1038/nn.4265 [Abstract]

‘Magneto’ manipulates behavior of freely moving mice
BY NICHOLETTE ZELIADT / 22 JUNE 2016
DOWNLOAD PDF
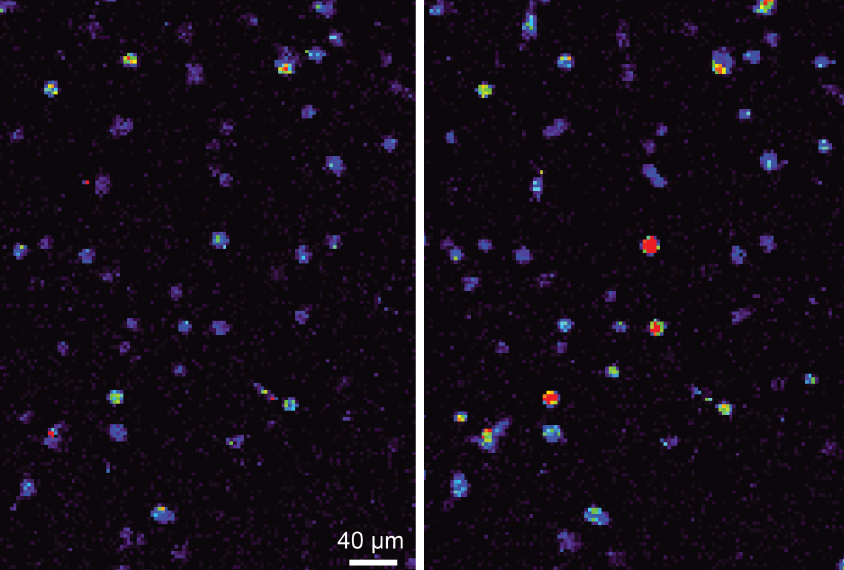 Laws of attraction: Neurons expressing a magnetically sensitive protein (right) show a spike in calcium levels when exposed to a magnet.
Laws of attraction: Neurons expressing a magnetically sensitive protein (right) show a spike in calcium levels when exposed to a magnet.
A modified protein allows researchers to use a magnet to switch on neurons anywhere in the brain in freely moving mice and zebrafish. The tool, described in May in Nature Neuroscience, could shed light on neural circuits underlying autism-like behaviors in animal models of the condition1.
Scientists can already turn neurons on and off at will with a technique called optogenetics that renders the cells sensitive to light. But that method requires surgically implanting a light source near the cells they want to manipulate.
The researchers rendered an ion channel in neurons called TPRV4 magnetically sensitive by fusing it to ferritin, a protein rich in iron. TPRV4 is ordinarily heat- and pressure-sensitive, but the researchers reasoned that, when attached to ferritin, it would also open in the presence of a magnetic field. Opening the channel causes calcium to flow into the cell, prompting it to fire.
Placing a magnet near cultured kidney cells expressing the protein, dubbed ‘Magneto,’ causes a calcium-sensitive fluorescent probe inside them to light up within seconds. And placing a magnet next to brain slices from mice that had been ‘infected’ by a virus carrying the Magneto gene causes neurons in the slices to fire. This firing stops when the tissue is bathed in a drug that blocks TPRV4.
Coiling on cue: Zebrafish embryos injected with Magneto coil defensively in the presence of a magnetic field.
The team also inserted the protein into neurons in the mouse striatum, an interior brain region that processes rewards and is difficult to target using optogenetics. Placing the mice in a magnetized chamber triggered firing of these neurons. Mice injected with Magneto spent more time in the magnetized chamber than in an adjacent non-magnetized area, suggesting that they experience a ‘reward’ when the magnet activates the neurons.
Magneto is likely to be still sensitive to temperature and pressure, making it hard to precisely control. But the researchers say that flaw may be fixable. – Spectrum News
IN CASE YOU EVER WONDERED WHY TEMPERATURE IS SUCH AN ISSUE WHEN IT COMES TO THE MRNA INJECTIONS…
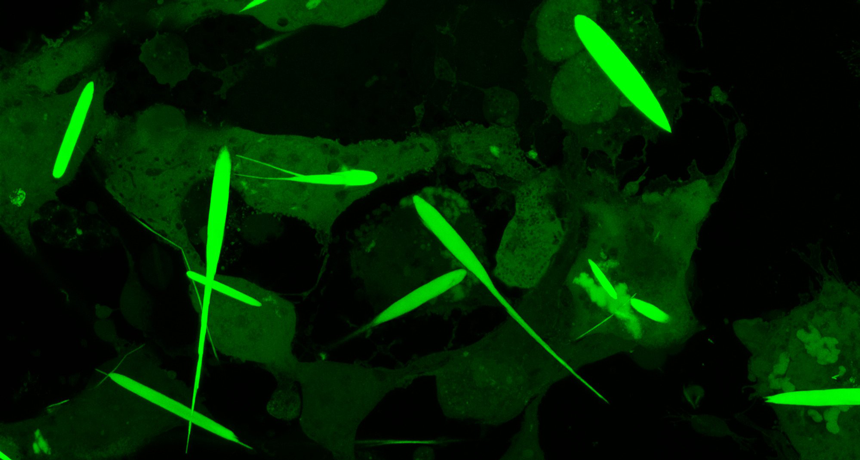
Like Magneto? Microcrystals give magnets superpower over living cells
These iron-rich protein crystals could be the future of how scientists study nerve cells
December 17, 2019 at 6:45 am
Imagine if you could control someone by using a magnet. It would be a bit like Magneto, the supervillain in X-Men. He can control anything magnetic. Even the iron inside someone’s body.
Controlling people with magnets sounds a little, well, wacky. But scientists have now done something close to that. They have engineered cells to make long, needle-like crystals rich in iron. Researchers can then use magnets to control cells containing these crystals.
Video recordings show these iron-rich crystals moving toward a strong magnet. The crystals pull the entire cell along with them.
Cui and her colleagues didn’t set out to give scientists superpowers like Magneto’s. Instead, their new protein crystals were designed to help scientists study which neurons control an animal’s movements and senses. The crystals provide something inside a cell that magnets can attract. This innovation fills a gap in the budding field of magnetogenetics (Mag-NEE-toh-jeh-NET-iks).
Scientists in this field genetically engineer cells so that they will respond to magnetic fields. Now researchers can remotely control specific neurons in the body using magnets. Those neurons could be ones that control how hungry an animal gets. Or they could be neurons that control leg muscles so a mouse starts running when a magnet is nearby.
Gaining magnetic control
A magnetic field can turn on neurons that contain proteins rich in iron. The field does this by heating or giving a mechanical push to those proteins.
Researchers had already been able to control neurons with light. That process is called optogenetics. To use it, scientists insert light-sensitive molecules into the neurons of living animals. The researchers can then turn the neurons on or off simply by shining a light on them. With this technique, neuroscientists have done some incredible things. They’ve made mice run in circles. They’ve even restored movement to an animal’s paralyzed leg.
But optogenetics has its downsides. Light, for example, can’t penetrate deeply into the body. There’s just too much bone, muscle and other tissue in the way. So researchers may implant optical fibers into the animal to deliver light to deep neurons. That makes the method cumbersome and even potentially dangerous.
The whole idea behind magnetogenetics is that you don’t have to implant anything, explains Jacob Robinson, who was not involved in the study. He’s a neuroengineer who works at Rice University in Houston, Texas.
Cells deep inside the body could be switched on with just a magnetic field. No fibers or surgery would be needed.
But there’s a snag. The only protein found naturally inside animal cells that’s even remotely magnetic is ferritin (FAIR-ih-tin). Each molecule can have as many as 4,500 atoms of iron. That may sound like a lot, but it’s not. The force that a magnet acting on ferritin generated would be only a billionth as strong as would be needed to turn on a neuron. So Cui’s team developed protein crystals that could carry enough iron to make their cells responsive to magnets.
Giant crystals with an iron heart
The team first extracted the gene to make ferritin from a microbe. They then made a circular piece of DNA that contained two human genes. Those genes make long, hollow crystals called inka-PAK4 (short for Inkabox-PAK4cat). The team introduced these circular pieces of DNA into human kidney cells that were growing in a petri dish. A day later, the first crystals appeared.
“When I first saw those crystals assemble in the cells by themselves, it was just amazing,” Cui recalls.
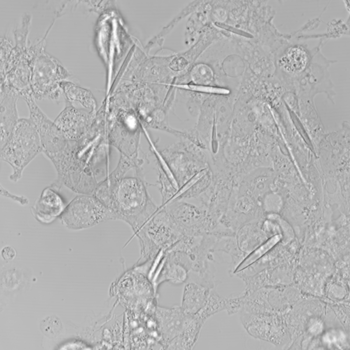
The crystals grew for three days until they were 45 millionths of a meter long. That’s about half the average thickness of a human hair. They’re the largest iron-containing protein crystals ever made in the lab — or in nature, Cui says. They were even longer than the cells they grew in. But the cells in which they formed never ripped. They just stretched to accommodate the crystals.
The researchers pried open the cells and removed the crystals. Then they loaded these with iron. The team estimates that it packed some 8 billion iron atoms into each crystal before inserting those crystals into human cells growing in a dish. Now they exposed the cells to a magnetic field and waited to see what would happen.
And the cells moved.
“The first time I actually saw [the cells] move toward the magnet, I was like, ‘Wow!’” Cui says.
Crystals started collecting close to the magnet. And the crystals pulled their cells with them. The team described this online September 25 in Nano Letters.
Robinson expressed excitement over this. “It’s an excellent step,” he said, “toward engineering cells to create their own magnetic nanoparticles.”
Scientists aren’t sure what will happen to the crystals afterward. But the cells have the genes for the crystals. So every cell reproduced from the original cells should be able to make the crystals, Cui says.
Iron not included
As promising as the results are, both Cui and Robinson emphasized that this isn’t the end.
“We still haven’t reached the goal,” Cui says.
Ideally, researchers would not need to first remove newly grown crystals to pack them full of the metal atoms. Instead, cells would enrich the crystals with iron as it built them. In fact, Cui’s group tried three different ways to get iron into its cells. They even drenched the cells in an iron-rich solution. Nothing worked.
Cells typically keep their iron levels low, Cui’s team notes. It’s estimated that cells naturally contain only 3 percent as much iron as the crystals would need to be effective.
We probably need to alter the cell’s outer membranes, Cui suspects. Then, she says, they might be able to transport more iron into a cell. Still, these magnetic crystals are a major leap forward in the young field of magnetogenetics. And the researchers are confident additional studies will overcome this iron-enrichment obstacle.
Published online 2020 Sep 22.
10.1021/acsanm.0c02048 PMCID: PMC7526334
And then we have this suspect:
2013, Oct 15
Biomedical applications of graphene and graphene oxide
Chul Chung, Young-Kwan Kim, Dolly Shin, Soo-Ryoon Ryoo, Byung Hee Hong, Dal-Hee Min
- PMID: 23480658
- DOI: 10.1021/ar300159f
Abstract
Graphene has unique mechanical, electronic, and optical properties, which researchers have used to develop novel electronic materials including transparent conductors and ultrafast transistors. Recently, the understanding of various chemical properties of graphene has facilitated its application in high-performance devices that generate and store energy. Graphene is now expanding its territory beyond electronic and chemical applications toward biomedical areas such as precise biosensing through graphene-quenched fluorescence, graphene-enhanced cell differentiation and growth, and graphene-assisted laser desorption/ionization for mass spectrometry. In this Account, we review recent efforts to apply graphene and graphene oxides (GO) to biomedical research and a few different approaches to prepare graphene materials designed for biomedical applications. Because of its excellent aqueous processability, amphiphilicity, surface functionalizability, surface enhanced Raman scattering (SERS), and fluorescence quenching ability, GO chemically exfoliated from oxidized graphite is considered a promising material for biological applications. In addition, the hydrophobicity and flexibility of large-area graphene synthesized by chemical vapor deposition (CVD) allow this material to play an important role in cell growth and differentiation. The lack of acceptable classification standards of graphene derivatives based on chemical and physical properties has hindered the biological application of graphene derivatives. The development of an efficient graphene-based biosensor requires stable biofunctionalization of graphene derivatives under physiological conditions with minimal loss of their unique properties. For the development graphene-based therapeutics, researchers will need to build on the standardization of graphene derivatives and study the biofunctionalization of graphene to clearly understand how cells respond to exposure to graphene derivatives. Although several challenging issues remain, initial promising results in these areas point toward significant potential for graphene derivatives in biomedical research.
Similar articles
- Bioapplications of graphene constructed functional nanomaterials.Gulzar A, Yang P, He F, Xu J, Yang D, Xu L, Jan MO.Chem Biol Interact. 2017 Jan 25;262:69-89. doi: 10.1016/j.cbi.2016.11.019. Epub 2016 Nov 20.PMID: 27876601 Review.
- Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications.Singh DP, Herrera CE, Singh B, Singh S, Singh RK, Kumar R.Mater Sci Eng C Mater Biol Appl. 2018 May 1;86:173-197. doi: 10.1016/j.msec.2018.01.004. Epub 2018 Feb 1.PMID: 29525091 Review.
- Rational design of carboxyl groups perpendicularly attached to a graphene sheet: a platform for enhanced biosensing applications.Bonanni A, Chua CK, Pumera M.Chemistry. 2014 Jan 3;20(1):217-22. doi: 10.1002/chem.201303582. Epub 2013 Dec 5.PMID: 24311348
- Graphene and graphene oxide as new nanocarriers for drug delivery applications.Liu J, Cui L, Losic D.Acta Biomater. 2013 Dec;9(12):9243-57. doi: 10.1016/j.actbio.2013.08.016. Epub 2013 Aug 16.PMID: 23958782
The best part seem to be these applications though:
- Covalent crosslinking of graphene oxide and carbon nanotube into hydrogels enhances nerve cell responses. Liu X , Miller Ii AL , Park S , Waletzki BE , Terzic A , Yaszemski MJ , Lu L .J Mater Chem B. 2016 Nov 21;4(43):6930-6941. doi: 10.1039/c6tb01722c. Epub 2016 Sep 20.PMID: 32263560
- Enhanced nerve cell proliferation and differentiation on electrically conductive scaffolds embedded with graphene and carbon nanotubes. Sun Y, Liu X, George MN, Park S, Gaihre B, Terzic A, Lu L.J Biomed Mater Res A. 2021 Feb;109(2):193-206. doi: 10.1002/jbm.a.37016. Epub 2020 Jul 28.PMID: 32441388
- Injectable Electrical Conductive and Phosphate Releasing Gel with Two-Dimensional Black Phosphorus and Carbon Nanotubes for Bone Tissue Engineering. Liu X, George MN, Li L, Gamble D, Miller Ii AL, Gaihre B, Waletzki BE, Lu L.ACS Biomater Sci Eng. 2020 Aug 10;6(8):4653-4665. doi: 10.1021/acsbiomaterials.0c00612. Epub 2020 Jul 9.PMID: 33455193
- [Research progress of neural tissue engineering based on electrically conductive carbon nanotube scaffold]. Xiang N, Wang G. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 Nov;25(11):1389-92.PMID: 22229201 Review. Chinese.
- Overview of Polyvinyl Alcohol Nanocomposite Hydrogels for Electro-Skin, Actuator, Supercapacitor and Fuel Cell. Wen N, Jiang B, Wang X, Shang Z, Jiang D, Zhang L, Sun C, Wu Z, Yan H, Liu C, Guo Z. Chem Rec. 2020 Aug;20(8):773-792. doi: 10.1002/tcr.202000001. Epub 2020 Mar 10.PMID: 32154653
Remote Neural Stimulation Using Magnetic Nanoparticles
Current Medicinal Chemistry, 2017
DOI: 10.2174/0929867323666160814000442
Abstract
Neural stimulation provides a means for scientists to investigate brain functions and neurological diseases. There is also mounting interest in using remote stimulation of neuronal circuits for brain-machine interfaces. In this review, we highlight recently developed technologies utilizing magnetic nanoparticles to generate heat or exert mechanical forces for remote control of brain circuits and compare these with conventional (electrical stimulation and drugs) and second-generation (ultrasound and light) techniques. We also present some of the challenges and progress in areas like genetics, nanoparticle synthesis and energy delivery devices to translate the use of these innovative nanoparticle-based platforms in research and clinical settings.
Magnetic Strategies for Nervous System Control
Michael G Christiansen 1, Alexander W Senko 2, Polina Anikeeva 2
Abstract
Magnetic fields pass through tissue undiminished and without producing harmful effects, motivating their use as a wireless, minimally invasive means to control neural activity. Here, we review mechanisms and techniques coupling magnetic fields to changes in electrochemical potentials across neuronal membranes. Biological magnetoreception, although incompletely understood, is discussed as a potential source of inspiration. The emergence of magnetic properties in materials is reviewed to clarify the distinction between biomolecules containing transition metals and ferrite nanoparticles that exhibit significant net moments. We describe recent developments in the use of magnetic nanomaterials as transducers converting magnetic stimuli to forms readily perceived by neurons and discuss opportunities for multiplexed and bidirectional control as well as the challenges posed by delivery to the brain. The variety of magnetic field conditions and mechanisms by which they can be coupled to neuronal signaling cascades highlights the desirability of continued interchange between magnetism physics and neurobiology.
Magnetic-Nanosensor-Based Virus and Pathogen Detection Strategies before and during COVID-19

Abstract
This review covers the literature of magnetic nanosensors for virus and pathogen detection before COVID-19. We review popular magnetic nanosensing techniques including magnetoresistance, magnetic particle spectroscopy, and nuclear magnetic resonance. Magnetic point-of-care diagnostic kits are also reviewed aiming at developing plug-and-play diagnostics to manage the SARS-CoV-2 outbreak as well as preventing future epidemics. In addition, other platforms that use magnetic nanomaterials as auxiliary tools for enhanced pathogen and virus detection are also covered. The goal of this review is to inform the researchers of diagnostic and surveillance platforms for SARS-CoV-2 and their performances.
NOVEMBER 30, 2020
Molecule that promotes muscle health when magnetised
by National University of Singapore

As people age, they progressively lose muscle mass and strength, and this can lead to frailty and other age-related diseases. As the causes for the decline remain largely unknown, promoting muscle health is an area of great research interest. A recent study led by the researchers from NUS has shown how a molecule found in muscles responds to weak magnetic fields to promote muscle health.
Led by Associate Professor Alfredo Franco-Obregón from the NUS Institute for Health Innovation and Technology (iHealthtech), the team found that a protein known as TRPC1 responds to weak oscillating magnetic fields. Such a response is normally activated when the body exercises. This responsiveness to magnets could be used to stimulate muscle recovery, which could improve the life quality for patients with impaired mobility, in an increasingly aging society.
“The use of pulsed magnetic fields to simulate some of the effects of exercise will greatly benefit patients with muscle injury, stroke, and frailty as a result of advanced age,” said lead researcher Assoc Prof Franco-Obregón, who is also from the NUS Department of Surgery.
The NUS research team collaborated with the Swiss Federal Institute of Technology (ETH) on this study, and their results were first published online in Advanced Biosystems on 2 September 2020. The work was also featured on the cover of the journal’s print edition on 27 November 2020.
Magnets and muscle health
The magnetic fields that the research team used to stimulate the muscle health were only 10 to 15 times stronger than the Earth’s magnetic field, yet still much weaker than a common bar magnet, raising the intriguing possibility that weak magnetism is a stimulus that muscles naturally interact with.
To test this theory, the research team first used a special experimental setup to cancel the effect of all surrounding magnetic fields. The researchers found that the muscle cells indeed grew more slowly when shielded from all environmental magnetic fields. These observations strongly supported the notion that the Earth’s magnetic field naturally interacts with muscles to elicit biological responses.
To show the involvement of TRPC1 as an antenna for natural magnetism to promote muscle health, the researchers genetically engineered mutant muscle cells that were unresponsive to any magnetic field by deleting TRPC1 from their genomes. The researchers were then able to reinstate magnetic sensitivity by selectively delivering TRPC1 to these mutant muscle cells in small vesicles that fused with the mutant cells.
In their previous studies, the researchers have shown that responses to such magnetic fields were strongly correlated to the presence of TRPC1, and it included the rejuvenation of cartilage by indirectly regulating the gut microbiome, fat burning and insulin-sensitivity via positive actions on muscle. The present study provided conclusive evidence that TRPC1 serves as a ubiquitous biological antenna to surrounding magnetic fields to modulate human physiology, particularly when targeted for muscle health.
Metabolic changes similar to those achieved with exercise have been observed in previous clinical trials and studies led by Assoc Prof Franco-Obregón. Encouraging benefits of using the magnetic fields to stimulate muscle cells have been found, with as little as 10 minutes of exposure per week. This tantalizing possibility, to improve muscle health without exercising, could facilitate recovering and rehabilitation of patients with muscle dysfunction.
Assoc Prof Franco-Obregón shared, “About 40 percent of an average person’s body is muscle. Our results demonstrate a metabolic interaction between muscle and magnetism which hopefully can be exploited to improve human health and longevity.”
This study represents a milestone in the understanding of how a key protein may developmentally react to magnetic fields.
Metabolic health such as weight, blood sugar levels, insulin, and cholesterol are strongly influenced by muscle health. As exercise is a strong modulator of metabolic diseases through the working of the muscles, and magnetic fields exert similar benefits of exercise, such magnetism may help patients who are unable to undertake exercise because of injury, disease, or frailty. As such, the NUS iHealthtech research team is now working to extend their study to reduce drug dependence for the treatment of diseases such as diabetes.
“We hope that our research can help alleviate side effects by reducing the use of drugs for disease treatment, and to improve the quality of life of the patients,” said Assoc Prof Franco-Obregón.
JANUARY 2021
A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Mice
Cite this: ACS Cent. Sci. 2021, 7, 1, 183–199Publication Date: January 5, 2021 https://doi.org/10.1021/acscentsci.0c01405
Copyright © 2021 The Authors. Published by American Chemical Society
“The development of a safe and effective SARS-CoV-2 vaccine is a public health priority. We designed subunit vaccine candidates using self-assembling ferritin nanoparticles displaying one of two multimerized SARS-CoV-2 spikes: full-length ectodomain (S-Fer) or a C-terminal 70 amino-acid deletion (SΔC-Fer). Ferritin is an attractive nanoparticle platform for production of vaccines, and ferritin-based vaccines have been investigated in humans in two separate clinical trials. We confirmed proper folding and antigenicity of spike on the surface of ferritin by cryo-EM and binding to conformation-specific monoclonal antibodies. After a single immunization of mice with either of the two spike ferritin particles, a lentiviral SARS-CoV-2 pseudovirus assay revealed mean neutralizing antibody titers at least 2-fold greater than those in convalescent plasma from COVID-19 patients. Additionally, a single dose of SΔC-Fer elicited significantly higher neutralizing responses as compared to immunization with the spike receptor binding domain (RBD) monomer or spike ectodomain trimer alone. After a second dose, mice immunized with SΔC-Fer exhibited higher neutralizing titers than all other groups. Taken together, these results demonstrate that multivalent presentation of SARS-CoV-2 spike on ferritin can notably enhance elicitation of neutralizing antibodies, thus constituting a viable strategy for single-dose vaccination against COVID-19.”

THE STUDY IS FINANCED BY MARK AND PRISCILLA ZUCKERBERG THROUGH BIOHUB!
- Corresponding Author
- Peter S. Kim – Department of Biochemistry & Stanford ChEM-H, Stanford University, Stanford, California 94305, United States; Chan Zuckerberg Biohub, San Francisco, California 94158, United States; http://orcid.org/0000-0001-6503-4541; Email: kimpeter@stanford.edu
- Authors
- Abigail E. Powell – Department of Biochemistry & Stanford ChEM-H, Stanford University, Stanford, California 94305, United States; http://orcid.org/0000-0001-6408-9495
- Kaiming Zhang – Department of Bioengineering & James H. Clark Center, Stanford University, Stanford, California 94305, United States; http://orcid.org/0000-0003-0414-4776
- Mrinmoy Sanyal – Department of Biochemistry & Stanford ChEM-H, Stanford University, Stanford, California 94305, United States
- Shaogeng Tang – Department of Biochemistry & Stanford ChEM-H, Stanford University, Stanford, California 94305, United States
- Payton A. Weidenbacher – Department of Biochemistry & Stanford ChEM-H, Stanford University, Stanford, California 94305, United States; Department of Chemistry, Stanford University, Stanford, California 94305, United States
- Shanshan Li – Department of Bioengineering & James H. Clark Center, Stanford University, Stanford, California 94305, United States
- Tho D. Pham – Department of Pathology, Stanford University, Stanford, California 94305, United States; Stanford Blood Center, Palo Alto, California 94304, United States
- John E. Pak – Chan Zuckerberg Biohub, San Francisco, California 94158, United States
- Wah Chiu – Department of Bioengineering & James H. Clark Center, Stanford University, Stanford, California 94305, United States; Chan Zuckerberg Biohub, San Francisco, California 94158, United States; Division of CryoEM and Bioimaging, SSRL, SLAC National Accelerator Laboratory, Menlo Park, California 94025, United States; http://orcid.org/0000-0002-8910-3078
- Notes
- The authors declare the following competing financial interest(s): A.E.P., P.A.W., and P.S.K. are named as inventors on a provisional patent application applied for by Stanford University and the Chan Zuckerberg Biohub on immunogenic coronavirus fusion proteins and related methods.
About
Ah, wait, Bill Gates is involved too!

Jul 30, 2020 · 4 min read
Chan Zuckerberg Initiative, Chan Zuckerberg Biohub, & the State of California Partner to Track COVID-19 Spread Statewide
California COVID Tracker is the First Statewide SARS-CoV-2 Tracking Program of Its Kind in the United States — Will Help Local Health Officials Better Map the VirusTags: COVID-19, CZ Biohub, Science
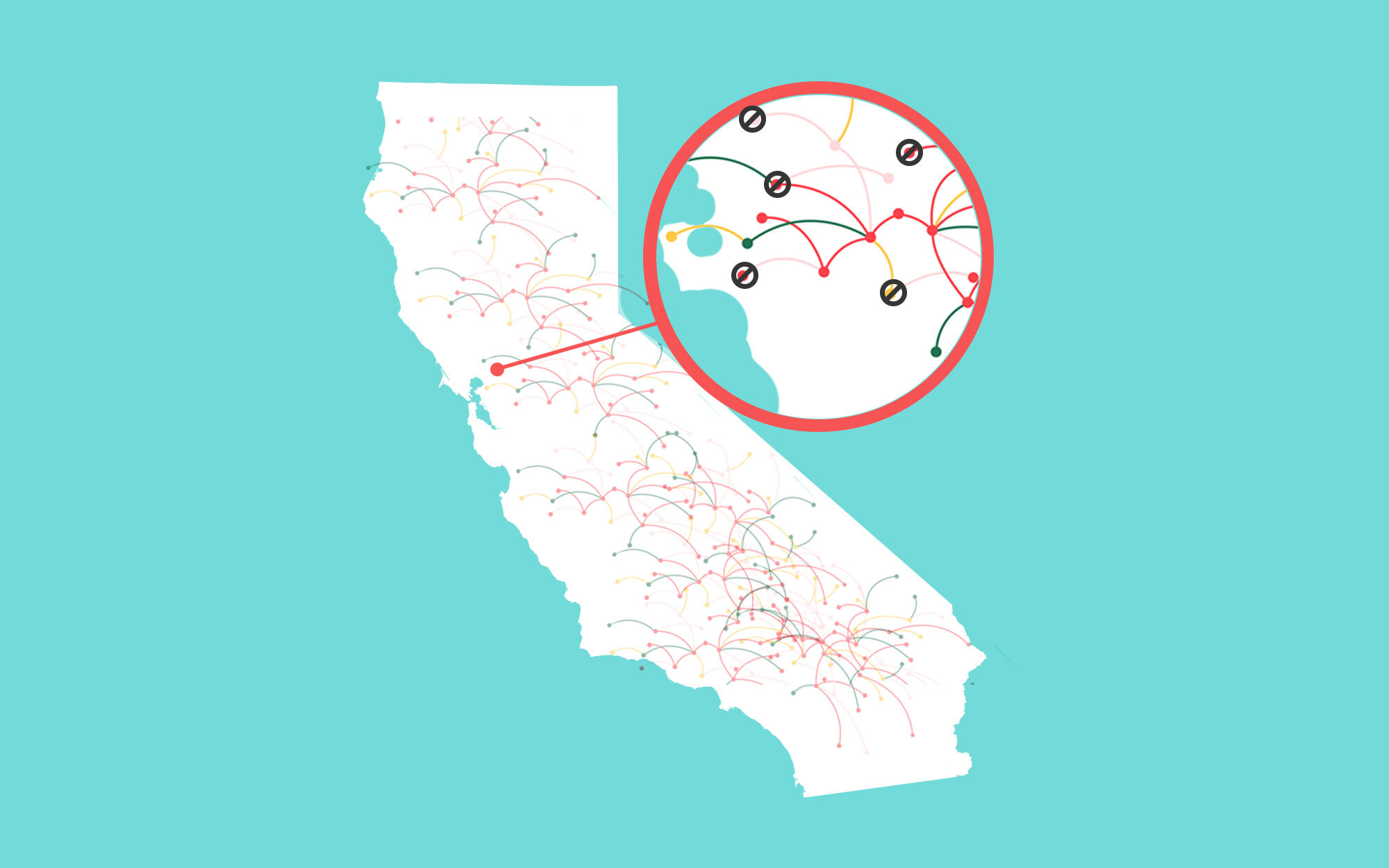
Whole genome sequencing allows scientists to track mutations of the SARS-CoV-2 virus, which typically happens every 2-3 transmissions. These mutations are key to helping public health officials trace transmission sources.
Today, the Chan Zuckerberg Biohub (CZ Biohub), in partnership with the Chan Zuckerberg Initiative (CZI), announced that it will provide free whole genome sequencing and analysis of the SARS-CoV-2 virus to all California Departments of Public Health (DPH) and California local health jurisdictions through a newly-launched effort called the California COVID Tracker. By rapidly tracing how and where the virus is changing and spreading across the state, the California COVID Tracker aims to provide actionable viral genomic data to local public health jurisdictions and help ensure transmission remains low while we await a vaccine.
Under this new partnership, any California DPH may ship positive COVID-19 samples to the CZ Biohub, which will provide sequencing, analysis, and interpretation support, with an emphasis on making data actionable for public health surveillance and response. By tracing the emergence of SARS-CoV-2 virus mutations, genomic epidemiology can offer insights such as estimating the number of undetected cases in a community, identifying clusters of linked transmission events, and detecting new introductions of SARS-CoV-2 into a given area or community.
Connected in this way to local public health labs and county public health departments, this type of actionable genomic epidemiology program is not currently available anywhere else in the United States. The CZ Biohub will also offer training in bioinformatics and data interpretation to public health partners throughout the state, including those interested in building or augmenting sequencing and analytic capacity within their own departments. The groups will also work closely with the Centers for Disease Control and Prevention’s newly-launched SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology and Surveillance (SPHERES) consortium.
“Public health officials need accurate, timely information about how COVID-19 is spreading to make decisions that will help protect people,” said CZI co-founders and co-CEOs Dr. Priscilla Chan and Mark Zuckerberg. “Using genome sequencing, researchers can create viral family trees to track how the virus is spreading to help inform policy decisions. We hope that broader sequencing coverage across California will empower local health jurisdictions to better understand transmission dynamics and the corresponding action needed in their communities.”
As part of this effort, the CZ Biohub will deposit SARS-CoV-2 sequences into public repositories for COVID-19 genomics, including GISAID and NCBI. CZ Biohub and CZI will provide tools and analysis support to help California DPHs overlay epidemiological and demographic information onto this genomic data to better understand local SARS-CoV-2 transmission.
“Through the California COVID Tracker, researchers, epidemiologists, software engineers, and data scientists from CZI and CZ Biohub are working to provide critical SARS-CoV-2 genomic data to California public health officials and the broader scientific community so they can make smart decisions about public health actions like contact tracing and intervention strategies,” said Joe DeRisi, PhD, Co-President of the CZ Biohub, who contributed to the identification of the SARS coronavirus in 2003. “These data become increasingly more powerful with broader participation. We invite interested public health officials and universities to partner with us in the fight against this unprecedented pandemic — these efforts will go a long way to protect our state from future spikes as we continue to fight this pandemic.”
The California COVID Tracker expands upon the ongoing partnership between CZI, the CZ Biohub, and UCSF, which has provided free COVID-19 testing to all 58 California Departments of Public Health. For more information on how to become involved in the California COVID Tracker, please visit covidtracker.czbiohub.org or email covidtracker@czbiohub.org.
###
About the Chan Zuckerberg Initiative
Founded by Dr. Priscilla Chan and Mark Zuckerberg in 2015, the Chan Zuckerberg Initiative (CZI) is a new kind of philanthropy that’s leveraging technology to help solve some of the world’s toughest challenges — from eradicating disease, to improving education, to reforming the criminal justice system. Across three core Initiative focus areas of Science, Education, and Justice & Opportunity, we’re pairing engineering with grant-making, impact investing, and policy and advocacy work to help build an inclusive, just and healthy future for everyone. For more information, please visit www.chanzuckerberg.com.
About the Chan Zuckerberg Biohub
The Chan Zuckerberg Biohub is a nonprofit research organization setting the standard for collaborative science, where leaders in science and technology come together to drive discovery and support the bold vision to cure, prevent or manage disease in our children’s lifetime. The CZ Biohub seeks to understand the fundamental mechanisms underlying disease and to develop new technologies that will lead to actionable diagnostics and effective therapies. The CZ Biohub is a regional research endeavor with international reach, where the Bay Area’s leading institutions — the University of California, Berkeley, Stanford University and the University of California, San Francisco — join forces with the CZ Biohub’s innovative internal team to catalyze impact, benefitting people and partnerships around the world. To learn more, visit CZBiohub.org.
LATEST RESEARCH UPDATES:
DECEMBER 2020
Magnetically controlled, hydrogel-based smart transformers
by Thamarasee Jeewandara , Phys.org
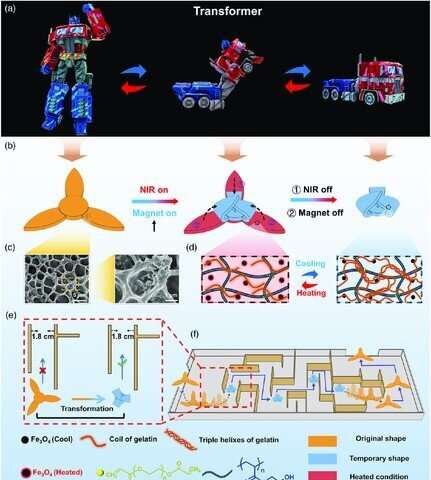
While the film “Transformers” introduced intelligent robots that morphed between shapes with multiple functionalities, researchers are developing intelligent soft transformers to significantly accelerate research applications in the lab. In a recent report now published in Advanced Intelligent Systems, Dachuan Zhang and a research team in materials science and chemical sciences in China, proposed a remotely controlled soft transformer based on a shape memory hydrogel system. The team obtained the hydrogel by embedding magnetite (Fe3O4) magnetic nanoparticles into a double network polymer structure of poly (N-(2-hydroxyethyl) acrylamide) containing gelatin.
The reversible coil-triple-helix transformation of the gelatin constituent imbued the hydrogel with shape memory and self-healing properties, while the magnetite nanoparticles gave photothermal heating and magnetic manipulation functions to deform the hydrogel for navigation in a magnetic field. The team could then restore the deformed shape via shape recovery using light irradiation. Zhang et al. remotely controlled the shape-memory processes through magnetically driven actuation and light-assisted shape memory. As proof of concept, they created a series of robots, including a hydrogel athlete that could do sit-ups, hydrogel transformers, a lotus in full bloom, and a hydrogel spacecraft that can be docked in air. The work will inspire the design and fabrication of new smart polymer systems with synchronized multiple functionalities.
Shape memory hydrogels
While the fictional transformers allowed hard robots to morph into any form including vehicles, soft transformers are of greater interest in fundamental research and applications in life sciences. In this work, Zhang et al. described a photothermally and magnetically controlled shape memory hydrogel. They combined a chemically crosslinked polymer and a reversibly crosslinked gelatin network embedded with magnetite nanoparticles to create a photothermal and flexible, self-healing construct that could be magnetically manipulated. Shape memory hydrogels (SMHs) have received increased attention as intelligent polymeric materials and researchers aim to remotely control such materials to establish diverse actuating behaviors.

For example, shape-memory polymers can fix temporary shapes and recover their architecture under external stimuli, with increasing interest across biomedical, textile, flexible electronics and data encryption disciplines. Magnetic nanoparticles are effective additives to introduce remotely controlled non-contact actuation. When hydrogels are illuminated with near-infrared (NIR) light, these magnetic nanoparticles will continuously convert light into heat, causing the hydrogel to be heated. This will cause reversible deformation of the hydrogel for applications as freely moving soft robots. This strategy will help promote the development of new shape memory hydrogel systems for applications as untethered robots.
Properties of shape memory hydrogels
Since shape memory hydrogels can stably and temporarily memorize their shape and recover the original shape perfectly under specific stimuli, the team conducted bending tests with the material, which they abbreviated as HG for its constituent polymers. They then immersed a sample in hot water (60 degrees Celsius) for 30 seconds to induce disaggregation to soften the hydrogel, removed it from the medium and recovered the shapes after re-immersing hydrogels in hot water (60 degrees Celsius). Zhang et al. conducted a series of controlled experiments to verify the factors affecting the shape memory performance of the hydrogel. As proof of concept, the team designed and developed a hydrogel flower to perfectly mimic the bloom of a lotus.

When the researchers introduced magnetite nanoparticles to form the HG-Fe3O4 hydrogel, the constituents could absorb and convert light to heat with light irradiation, causing the temperature of the hydrogel to increase. During light-to-heat conversion, the material achieved photo-activated self-healing. To demonstrate this phenomenon, the team created a HG-Fe3O4 hydrogel space station under a magnetic field and applied NIR to irradiate the connectors and dock the spacecraft-like construct with a space station-like connector to realize self-healing and reconnection in air.
Recovering shapes through photothermal effects and remotely controlling shape memory processes
The team could only achieve shape recovery for the HG-hydrogel by regulating the temperature to a specific value, in the absence of magnetite nanoparticles. The addition of magnetite conferred magnetic properties to the HG-Fe3O4 hydrogel to allow remotely controlled shape memory recovery cycles. As proof of concept, the team developed a shape-transition robot in the form of a hydrogel athlete to deform from 2-D to 3-D. In the absence of NIR and the presence of a magnet, the hydrogel athlete could ‘push up’ quickly, then recover its shape to the flat conformation on removal of the magnet. In the second setup, they turned-on NIR and lifted the hydrogel athlete with a magnet, then kept the magnet on for two minutes while switching off the NIR to allow the athlete to cool down. The team froze this gesture for a timeframe after which they allowed the robot to return to its original position by turning-on the NIR again. This technique can be used to develop soft grippers that are advantageous for applications as surgical robots in translational research.

Abstract

Remote control of cells and single molecules by magnetic nanoparticles in nonheating external magnetic fields is a perspective approach for many applications such as cancer treatment and enzyme activity regulation. However, the possibility and mechanisms of direct effects of small individual magnetic nanoparticles on such processes in magneto-mechanical experiments still remain unclear. In this work, we have shown remote-controlled mechanical dissociation of short DNA duplexes (18–60 bp) under the influence of nonheating low-frequency alternating magnetic fields using individual 11 nm magnetic nanoparticles.
The developed technique allows (1) simultaneous manipulation of millions of individual DNA molecules and (2) evaluation of energies of intermolecular interactions in short DNA duplexes or in other molecules.
Finally, we have shown that DNA duplexes dissociation is mediated by mechanical stress and produced by the movement of magnetic nanoparticles in magnetic fields, but not by local overheating.
The presented technique opens a new avenue for high-precision manipulation of DNA and generation of biosensors for quantification of energies of intermolecular interaction.
MAY 18, 2021
New Material Could Create ‘Neurons’ and ‘Synapses’ for Computers
via University of Groningen
Classic computers use binary values (0/1) to perform. By contrast, our brain cells can use more values to operate, making them more energy-efficient than computers. This is why scientists are interested in neuromorphic (brain-like) computing. Physicists from the University of Groningen have used a complex oxide to create elements comparable to the neurons and synapses in the brain using spins, a magnetic property of electrons. Their results were published on 18 May in the journal Frontiers in Nanotechnology.
Thin films
The operation of our brains can be simulated in computers, but the basic architecture still relies on a binary system. That is why scientist look for ways to expand this, creating hardware that is more brain-like, but will also interface with normal computers. ‘One idea is to create magnetic bits that can have intermediate states’, says Tamalika Banerjee, Professor of Spintronics of Functional Materials at the Zernike Institute for Advanced Materials, University of Groningen. She works on spintronics, which uses a magnetic property of electrons called ‘spin’ to transport, manipulate and store information.
In this study, her PhD student Anouk Goossens, first author of the paper, created thin films of a ferromagnetic metal (strontium-ruthenate oxide, SRO) grown on a substrate of strontium titanate oxide. The resulting thin film contained magnetic domains that were perpendicular to the plane of the film. ‘These can be switched more efficiently than in-plane magnetic domains’, explains Goossens. By adapting the growth conditions, it is possible to control the crystal orientation in the SRO. Previously, out-of-plane magnetic domains have been made using other techniques, but these typically require complex layer structures.
Magnetic anisotropy
Schematic of the proposed device structure for neuromorphic spintronic memristors. The write path is between the terminals through the top layer (black dotted line), the read path goes through the device stack (red dotted line). The right side of the figure indicates how the choice of substrate dictates whether the device will show deterministic or probabilistic behavior. | Illustration Banerjee group
The magnetic domains can be switched using a current through a platinum electrode on top of the SRO. Goossens: ‘When the magnetic domains are oriented perfectly perpendicular to the film, this switching is deterministic: the entire domain will switch.’ However, when the magnetic domains are slightly tilted, the response is probabilistic: not all the domains are the same, and intermediate values occur when only part of the crystals in the domain have switched.
By choosing variants of the substrate on which the SRO is grown, the scientists can control its magnetic anisotropy. This allows them to produce two different spintronic devices. ‘This magnetic anisotropy is exactly what we wanted’, says Goossens. ‘Probabilistic switching compares to how neurons function, while the deterministic switching is more like a synapse.’
The scientists expect that in the future, brain-like computer hardware can be created by combining these different domains in a spintronic device that can be connected to standard silicon-based circuits. Furthermore, probabilistic switching would also allow for stochastic computing, a promising technology which represents continuous values by streams of random bits. Banerjee: ‘We have found a way to control intermediate states, not just for memory but also for computing.’
Reference:
A.S. Goossens, M.A.T. Leiviskä and T. Banerjee: Anisotropy and Current Control of Magnetization in SrRuO3/SrTiO3 Heterostructures for Spin-Memristors. Frontiers in Nanotechnology 18 May 2021
Above: Video abstract of an original research “Magnetically controlled protein nanocontainers as a drug depot for the hemostatic agent” published in the open access journal Nanotechnology, Science and Applications by Prilepskii, Schekina and Vinogradov.
Purpose: Currently, there is a number of successfully implemented local hemostatic agents for external bleedings in forms of wound dressings and other topical materials. However, little has been done in the field of intravenous hemostatic agents. Here, we propose a new procedure to fabricate biocompatible protein nanocontainers (NCs) for intravenous injection allowing magneto-controllable delivery and short-term release of the hemostatic agent ϵ-aminocaproic acid (EACA). Methods: The nanocontainers were synthesized by the desolvation method from bovine serum albumin (BSA) using methanol without any further crosslinking. Polyethylene glycol (PEG) was used both as a stabilization agent and for size control. Characterization of nanocontainers was performed by the transmission and scanning electron microscopy, dynamic light scattering, X-ray diffraction, and FTIR spectroscopy. Cytotoxicity was estimated using MTT assay. The dopant release from nanocontainers was measured spectrophotometrically using rhodamine B as a model molecule. The specific hemostatic activity was assessed by analyzing clot lysis and formation curve (CloFAL). Moreover, the ability for magneto targeting was estimated using the original flow setup made of a syringe pump and silicon contours.
Results: Fabricated nanocontainers had an average size of 186±24 nm and were constructed from building blocks–nanoparticles with average size ranged from 10 to 20 nm. PEG shell was also observed around nanocontainers with thickness 5–10 nm. NCs were proved to be completely non-cytotoxic even at concentrations up to 8 mg BSA/mL. Uptake capacity was near 36% while release within the first day was 17%. The analysis of the CloFAL curve showed the ability of NCs to inhibit the clot lysis successfully, and the ability of magneto targeting was confirmed under flow conditions.
Conclusion: The ability of synthesized NCs to deliver and release the therapeutic drug, as well as to accumulate at the desired site under the action of the magnetic field was proved experimentally.
Read the full paper HERE
Full article: https://doi.org/10.1152/ajpgi.00233.2017
The problem with magnetofection is that it keeps killing lab animals and it’s not recommended or forbidden on humans. Or it used to be, for almost 10 years before Covidiocracy.
OTHER RESOURCES:
https://www.embopress.org/doi/pdf/10.15252/embj.201797177
https://pubmed.ncbi.nlm.nih.gov/31552740/
https://pubmed.ncbi.nlm.nih.gov/28960485/
https://pubmed.ncbi.nlm.nih.gov/20553812/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7068712/
I will add more resources and refine this in the near future, but I think the case is made and it’s more than solid.
PS: Connect the dots with the earlier post on 5G as a wireless power grid
To be continued?
Our work and existence, as media and people, is funded solely by our most generous supporters. But we’re not really covering our costs so far, and we’re in dire needs to upgrade our equipment, especially for video production.
Help SILVIEW.media survive and grow, please donate here, anything helps. Thank you!
! Articles can always be subject of later editing as a way of perfecting them

You can even eat some of them.
CLICK HERE




